Animal Mycoplasmas
General characteristics
Mycoplasmas are the simplest self-replicating prokaryotes that are distinguished from ordinary bacteria by a complete lack of a cell wall, a small cell and genome size, and a low genomic GC content. Further distinguishing properties occurring in most species are the presence of cholesterol in the plasma membrane and UGA codon usage for tryptophan (stop codon in other bacteria).
Taxonomically, mycoplasmas are classified in the class Mollicutes (L mollis– soft, L cutis– skin), which is the only class of phylum Mycoplasmatota, commonly referred to as mycoplasmas or mollicutes. The class Mollicutes is highly divers comprising four orders, five families, nine genera, and more than 200 known species that have been detected in or isolated from humans, vertebrate animals (mammals, birds, reptiles, and fish), invertebrates, and plants. Almost all animal mycoplasmas (mollicutes that have been isolated/detected from/in vertebrate animals) are members of genera Mycoplasma, Ureaplasma, and Acholeplasma. Further details are provided at Taxonomy.
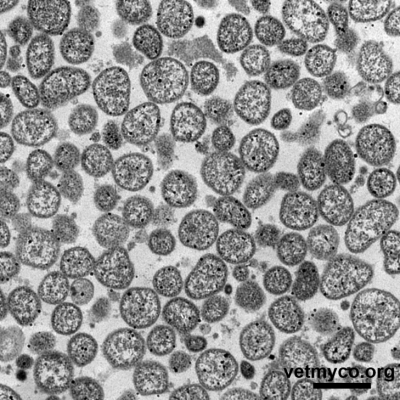
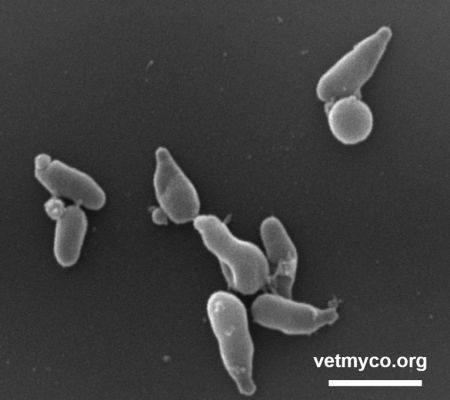
Since mycoplasmas are only bounded by a cell membrane, their cell morphology is pleomorphic varying from a dominating spherical to coccoid shape (Fig. 1) to pear- and flask-shaped cells (Fig. 2), filaments and helical morphology (the latter is characteristic for arthropod- and plant-infecting Spiroplasma). Although considered non-motile, some flask-shaped Mycoplasma species are capable of gliding on solid surfaces whereas members of genus Spiroplasma show rotary motility.
Metabolism
Mycoplasmas are evolutionary descendants of gram-positive bacteria from which they have evolved by significant genome reduction. As a result of this degenerative evolution, mycoplasmas possess limited anabolic and metabolic capabilities and maintain commensal or intimate parasitic lifestyles depending on nutrients from their host environments.
Based on their ability to metabolize carbohydrates for energy production, mycoplasmas are divided into fermentative and non-fermentative organisms. While members of the fermentative group convert carbohydrates such as glucose into the organic acid pyruvate (accompanied with release of free energy to form ATP), several species of the non-fermentative group possess the arginine dehydrolase pathway resulting in the production of ornithine, ATP, and ammonia (which causes an alkaline pH). Some mycoplasmas metabolize neither sugars nor arginine but are capable of oxidizing organic acids (lactate, pyruvate) to acetate. Species of genus Ureaplasma possess a unique ATP synthesis system coupled to urea hydrolysis by a very potent urease.
Diseases
Several animal mycoplasma species are considered commensals that are only sporadically associated with pathological conditions, while others are well recognized as opportunistic or primary pathogens. Animal mycoplasmas usually exhibit a rather strict host and tissue specificity with predilection for mucous surfaces of the respiratory and urogenital tract, the eyes, the mammary gland, the joints, and serous membranes. Pathogenic animal mycoplasmas are not considered highly virulent per se and mostly cause mildly to moderately severe, slowly progressive, chronic infections. In general, mycoplasma diseases display major elements of immunopathology with sometimes wide-ranging complications and sequelae. Most important and well recognized mycoplasma diseases are those affecting i) livestock and poultry which are responsible for substantial economic losses, ii) laboratory rodents causing effects on the validity of research data, and iii) wildlife contributing to population decline (Tab. 1). However, several animal mycoplasmas also play an etiologic role as opportunists in diseases of their hosts (including livestock, poultry, companion animals, and wildlife) although reasons and risk factors for disease development and progression are largely unknown in many cases. Further details on diseases associated with or caused by mycoplasmas are provided in each Mycoplasma Profile.
Table 1: Examples of important and well recognized diseases of livestock, poultry, laboratory rodents, and wildlife associated with or caused by animal mycoplasmas
| Main hosts | Mycoplasma species | Diseases |
| cattle | Contagious Bovine Pleuropneumonia (CBPP) Bovine Respiratory Disease (BRD), mastitis, arthritis, otitis | |
goats
sheep, goats | Mycoplasma capricolum ssp. capripneumoniae | Contagious Caprine Pleuropneumonia (CCPP) mastitis, arthritis, pneumonia, keratoconjunctivitis, septicemia Contagious Agalactia (CA) atypical pneumonia Infectious Keratoconjunctivitis (IKC) |
| swine | Enzootic Pneumonia (EP), MIRD, PRDC polyserositis, arthritis arthritis porcine eperythrozoonosis | |
chicken, turkey
turkey | Chronic Respiratory Disease (CRD), infectious sinusitis infectious synovitis airsacculitis, synovitis, reduced hatchability | |
| mice, rats | Mycoplasma pulmonis | Murine Respiratory Mycoplasmosis (MRM) |
wild song birds tortoises alligators | conjunctivitis epidemics Upper Respiratory Disease (URD) lethal pneumonia, polyserositis, pericarditis, and polyarthritis |
Pathogenicity
In mycoplasmas, mechanisms of pathogenicity and determinants of virulence are still largely unknown. However, several mechanisms of pathogenicity have been proposed including attachment to host cells, the production of capsular polysaccharides, intracellular localization, biofilm formation, antigenic variation, competition for biosynthetic precursors, uptake of high-energy compounds and host metabolites, the release of toxic by-products of metabolism such as hydrogen peroxide, the release of ammonia, Ig protease activity, and the induction of pathologic host immune reactions and inflammatory responses. Further details are provided at Pathogenicity.
Epidemiology
Mycoplasmas are transmitted between animals by direct contact, often through the airborne route, but also via milk in case of mammary gland infection, or via the genital route by mating, artificial insemination or orogenital contact. Mycoplasmas may also be transmitted vertically from an infected or colonized female to the egg, fetus or neonate. Epidemiological characteristics and transmission modes of mycoplasma infections have been variously evaluated by employing genotyping methods such as multi-locus variable number of tandem repeats (VNTR) analysis (MLVA), multi-locus sequence typing (MLST), and genome wide typing methods like core genome MLST (cgMLST) for intra-species differentiation allowing tracing sources and routes of infections.
Laboratory diagnosis
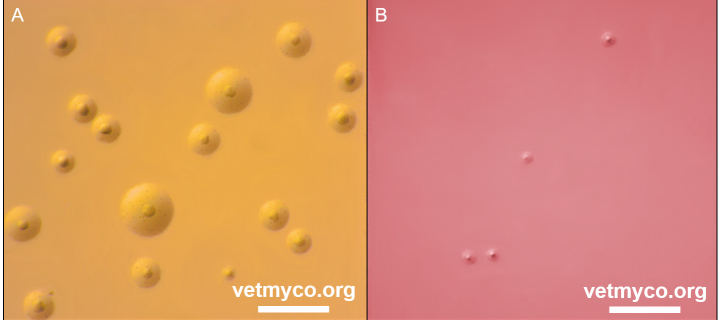
Laboratory diagnosis of mycoplasma infections is commonly achieved by conventional cultivation procedures in which most mycoplasmas produce colonies with characteristic fried egg morphology (Fig. 3), or by direct PCR for the detection of uncultivable mycoplasmas, highly fastidious species or mycoplasmas causing diseases of high veterinary importance. Cultivation is usually followed by antigenic or genetic identification of mycoplasma isolates. Antigenic identification tests such as membrane filtration dot immunobinding or colony epi-immunofluorescence depend on specific antisera to each individual mycoplasma species. Genetic identification of mycoplasma isolates is based on universal PCRs targeting the 16S rRNA gene, the 16S-23S intergenic spacer region or the rpoB gene followed by sequence or amplicon analyses. Most recently, MALDI-ToF mass spectrometry proved to be an excellent method for the identification and differentiation of all animal mycoplasmas. Further details are provided at Laboratory Diagnosis.
Fig. 3. (A) Mycoplasma bovis PG45T colonies on modified Hayflick’s agar exhibiting characteristic fried egg morphology, and (B) Ureaplasma cati F2T on U4 agar producing very tiny colonies characteristic for all members of genus Ureaplasma. Note a colour change of solid medium from original orange (U4 agar) to reddish based on release of ammonia resulting from hydrolysis of urea creating an alkaline pH. Bar, 1 mm. Credits: Joachim Spergser (Vetmeduni Vienna)
Treatment, prevention and control
Since mycoplasmas lack a cell wall, they are naturally resistant to antibiotics that inhibit cell wall synthesis such as β-lactams (penicillins, cephalosporins) and glycopeptides. Mycoplasmas are also intrinsically resistant to sulfonamides/trimethoprim, polymixins, and rifampicin. The most active and widely used antimicrobial agents in animals against mycoplasma infections are tetracyclines, macrolides, lincosamides, pleuromutilins, phenicols and fluoroquinolones. However, several mycoplasmas appear to be able to evade antimicrobial therapy possibly by forming biofilms, intracellular sequestration or localization in tissues where antimicrobials do not reach therapeutic concentration levels. In addition, reduced in vitro susceptibility levels or acquired resistance to several antimicrobials applied to treat mycoplasma infections have been reported in field isolates of pathogenic Mycoplasma species of veterinary interest.
Control measures to prevent introduction, transmission and spread of mycoplasmas are critical for those causing diseases with socio-economic impact in livestock. Control measures may include prevention of introducing mycoplasmas into herds by pre-testing animals for restocking and implementing quarantine measures, good farming practice, cleaning and disinfection, isolation and treatment of infected animals, etc.
Numerous vaccines have been developed to prevent mycoplasma diseases in animals, but their efficacy is sometimes doubtful or imperfectly evaluated. In general, commercially available vaccines, either inactivated or live attenuated, have shown to reduce losses and cost of treatment, and to improve performance, but failed to eliminate mycoplasmas from infected herds. Consequently, improved, safe and effective vaccines that provide long-term protective immunity are urgently required.
References
- Calcutt, M.J., Lysnyansky, I., Sachse, K., Fox, L.K., Nicholas, R.A.J. and R.D. Ayling. 2018. Gap analysis of Mycoplasma bovis disease, diagnosis and control: An aid to identify future development requirements. Transbound. Emerg. Dis. 65 Suppl. 1: 91-109.
- Citti, C. and R. Rosengarten. 1997. Mycoplasma genetic variation and its implication for pathogenesis. Wien. Klin. Wochenschr. 14-15: 562–568.
- Citti, C., Nouvel, L.X and E. Baranowski. 2010. Phase and antigenic variation in mycoplasmas. Future Microbiol. 5: 1073–1085.
- Citti, C. and A. Blanchard. 2013. Mycoplasmas and their host: emerging and re-emerging minimal pathogens. Trends Microbiol. 21: 196–203.
- Razin, S., Yogev, D. and Y. Naot.1998. Molecular biology and pathogenicity of mycoplasmas. Microbiol. Mol. Biol. Rev. 62: 1094–1156.
- Rocha, E.P. and A. Blanchard. 2002. Genomic repeats, genome plasticity and the dynamics of Mycoplasma evolution. Nucleic Acids Res. 30: 2031– 2042.
- Rosengarten, R., Citti, C., Glew, M., Lischewski, A., Droesse, M., Much, P., Winner, F., Brank, M. and J. Spergser. 2000. Host-pathogen interactions in mycoplasma pathogenesis: Virulence and survival strategies of minimalist prokaryotes. Int. J. Med. Microbiol. 290: 15–25.
- Rosengarten, R., Citti, C., Much, P., Spergser, J., Droesse, M. and M. Hewicker-Trautwein. 2001. The changing image of mycoplasmas: from innocent bystanders to emerging and reemerging pathogens in human and animal diseases. In: Mühldorfer, I. and K.P. Schäfer (eds.), Emerging Bacterial Pathogens, Contrib. Microbiol. Vol. 8, S. Karger, Switzerland.
- Rottem, S. 2003. Interaction of mycoplasmas with host cells. Physiol. Rev. 83: 417–432.
- Sirand-Pugnet, P., Citti, C., Barre, A. and A. Blanchard. 2007. Evolution of Mollicutes: down a bumpy road with twists and turns. Res. Microbiol. 158: 754–766.
- Spergser, J., Hess, C., Loncaric, I. and A.S. Ramírez. 2019. Matrix-assisted laser desorption ionization-time of flight mass spectrometry is a superior diagnostic tool for the identification and differentiation of mycoplasmas isolated from animals. J. Clin. Microbiol. doi: 10.1128/JCM.00316-19.
- Waites, K.B., Katz, B. and R.L. Schelonka. 2005. Mycoplasmas and ureaplasmas as neonatal pathogens. Clin. Microbiol. Rev. 18: 757–789.
- Zimmerman, C.-U. 2014. Current insights into phase and antigenic variation in mycoplasmas. In: Browning, G. and C. Citti (eds.). Mollicutes: Molecular biology and pathogenesis. Caister Academic Press, UK.