Taxonomy of animal mycoplasmas
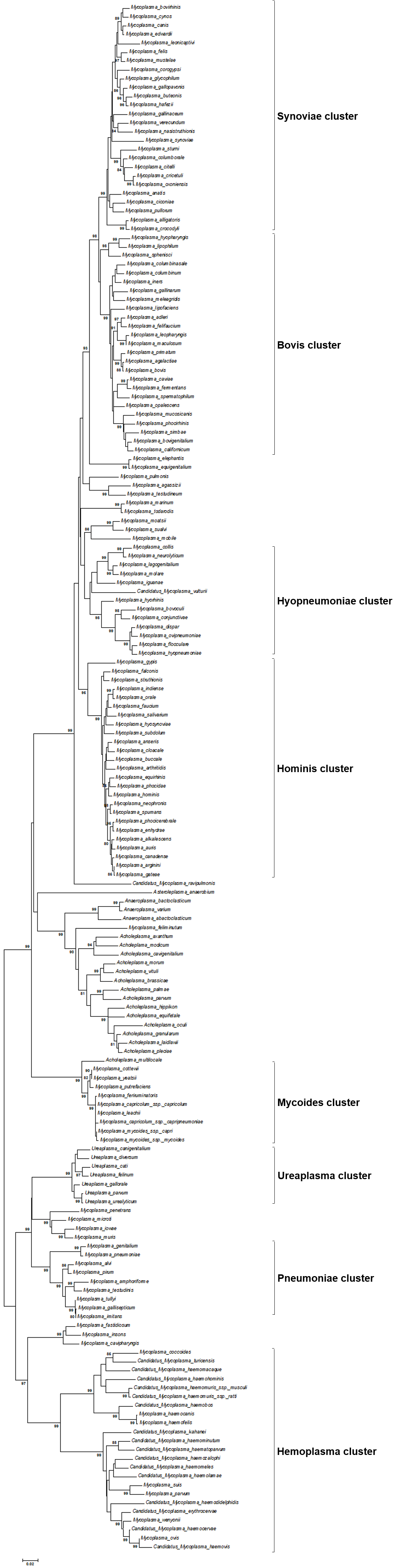
Mycoplasmas are classified in the class Mollicutes (L mollis– soft, L cutis– skin) which is the only class of phylum Mycoplasmatota, and distinguished from ordinary bacteria by a complete lack of a cell wall, a small cell and genome size, and a low genomic GC content. The class Mollicutes comprises four orders, five families, and 9 genera that have been characterised based on their morphology, growth temperature, sterol requirements, host specificity, cultivability, and oxygen sensitivity. Currently the class Mollicutes contains more than 200 known species. Almost all mycoplasmas that have been isolated from or detected in vertebrate animals are members of genera Mycoplasma, Ureaplasma or Acholeplasma (Tab. 1). All species of genera Haemobartonella and Eperythrozoon have been reclassified as hemotropic Mycoplasma species (Fig. 1).
Table 1: Taxonomy of animal and human mycoplasmas (class Mollicutes)
| Order | Family | Genus | Species* | Hosts (number of mollicutes species) |
| Mycoplasmatales | Mycoplasmataceae | Mycoplasma Ureaplasma | 159 9 | humans (12), animals (147) humans (2), animals (7) |
| Acholeplasmatales | Acholeplasmataceae | Acholeplasma | 13 | animals, rarely humans |
| Anaeroplasmatales | Anaeroplasmataceae | Anaeroplasma Asteroleplasma | 4 1 | bovine/ovine rumen bovine/ovine rumen |
*currently known mollicutes species that have been isolated from humans and/or animals including effectively but not validly published species and Candidatus species (per 01/06/2022)
The current taxonomy of Mollicutes relies on a combination of phenotypic features and phylogenetic assignments based on gene sequence analyses. Phenotypic criteria for classification include cultural, morphological, chemotaxonomic, serological as well as metabolic and enzymatic properties. Furthermore, mass spectrometry has recently been proven to be a powerful and supportive tool for species identification and differentiation, hence for the taxonomic resolution of mycoplasmas. Among these phenotypic differentia, serological characteristics have been widely applied for species identification and definition of novel species. However, serological tests do not always allow discrimination and identification since interspecies cross-reactivity and serological heterogeneity within a given species have shown to occur. Moreover, the taxonomic value of metabolic and enzymatic tests is limited and only enable the assignment of a mollicute isolate to either the genus level or a metabolic group such as the glucose-, arginine- or urea utilizers. These deficiencies of phenotypic classification have only been partially compensated by utilizing the 16S rRNA gene as a universal molecular marker for the taxonomic placement of a mycoplasma isolate to the genus level or a phylogenetic group or cluster.
In general, phylogenetic trees based on 16S rRNA gene analyses separate the order Mycoplasmatales into eight main phylogenetic clusters, namely the i) Bovis, ii) Synoviae, iii), Hominis, iv) Hyopneumoniae, v) Mycoides, vi) Pneumoniae, vii) Hemoplasma, and viii) Ureaplasma cluster (Fig. 1). However, the utility of the 16S rRNA gene as solitary genetic marker for species identification is limited since high interspecies sequence similarities (≥ 97%) among several mycoplasma species have been observed. Consequently, additional genetic markers such as the 16S-23S intergenic spacer sequence and the rpoB gene, both exhibiting high interspecies and low intraspecies diversity, have been applied. Analyses of further housekeeping genes may also be performed to substantiate phylogenetic position and taxonomic classification of a given mycoplasma isolate.
Moreover, phylogenomic data deduced from complete genome sequences strongly contribute to the taxonomy of Mollicutes. Genomic comparison and calculation of average nucleotide identity (ANI), tetra nucleotide signature correlation index (TETRA), average amino acid identity (AAI), and in silico digital DNA-DNA hybridization (dDDH) values are currently used as additional criteria for species delineation and taxonomic classification of mycoplasma isolates.
Fig. 1. Maximum likelihood tree showing the phylogenetic positions of animal mycoplasmas including members of orders Mycoplasmatales, Acholeplasmatales, and Anaeroplasmatales, based on 16S rRNA gene sequences. Separation of order Mycoplasmatales into eight main phylogenetic clusters is indicated. Numbers at nodes represent bootstrap confidence values (1000 replications). Only values > 80% are shown. Bar, number of substitutions per nucleotide position. Credits: Joachim Spergser (Vetmeduni Vienna)
References
- Balish, M., Bertaccini, A., Blanchard, A., Brown, D., Browning, G., Chalker, V., Frey, J., Gasparich, G., Hoelzle, L., Knight, T., Knox, C., Kuo, C.-H., Manso-Silván, L., May, M., Pollack, J.D., Ramírez, A.S., Spergser, J., Taylor-Robinson, D., Volokhov, D. and Y. Zhao. 2019. Recommended rejection of the names Malacoplasma gen. nov., Mesomycoplasma gen. nov., Metamycoplasma gen. nov., Metamycoplasmataceae fam. nov., Mycoplasmoidaceae fam. nov., Mycoplasmoidales ord. nov., Mycoplasmoides gen. nov., Mycoplasmopsis gen. nov. [Gupta, Sawnani, Adeolu, Alnajar and Oren 2018] and all proposed species comb. nov. therein. Int. J. Syst. Evol. Microbiol. doi: 10.1099/ijsem.0.003632.
- Brown, D.R., Whitcomb, R.F. and J.M. Bradbury. 2007. Revised minimal standards for description of new species of the class Mollicutes (division Tenericutes). Int. J. Syst. Evol. Microbiol. 57: 2703–2719.
- Brown, D.R., May, M., Bradbury, J.M. and K.E. Johansson. 2018. Mollicutes. In: Whitman, W.B., Rainey, F., Kämpfer, P., Trujillo, M., Chun, J., DeVos, P., Hedlund, B. and S. Dedysh (eds), Bergey’s Manual of Systematics of Archaea and Bacteria. John Wiley & Sons, Inc., in association with Bergey’s Manual Trust.
- Johansson, K.E., Heldtander, M.U. and B. Pettersson. 1998. Characterization of mycoplasmas by PCR and sequence analysis with universal 16S rDNA primers. Methods Mol. Biol. 104: 145–165.
- Neimark, H., Johansson, K.-E., Rikihisa, Y. and J.G. Tully. 2001. Proposal to transfer some members of the genera Haemobartonella and Eperythrozoon to the genus Mycoplasma with descriptions of ‘Candidatus Mycoplasma haemofelis’, ‘Candidatus Mycoplasma haemomuris’, ‘Candidatus Mycoplasma haemosuis’ and ‘Candidatus Mycoplasma wenyonii’. Int. J. Syst. Evol. Microbiol. 51: 891–899.
- May, M. and D.R. Brown. 2019. International Committee on Systematics of Prokaryotes, Subcommittee on the taxonomy of Mollicutes. Minutes of the closed meeting, 8 July 2018, Portsmouth, New Hampshire, USA. Int. J. Syst. Evol. Microbiol. 69: 2169–2171.
- Pettersson, B., Tully, J.G., Bölske, G. and K.E. Johansson. 2000. Updated phylogenetic description of the Mycoplasma hominis cluster (Weisburg et al., 1989) based on 16S rDNA sequences. Int. J. Syst. Evol. Microbiol. 50: 291–301.
- Volokhov, D.V., Simonyan, V., Davidson, M.K and V.E. Chizhikov. 2012. RNA polymerase beta subunit (rpoB) gene and the 16S-23S rRNA intergenic transcribed spacer region (ITS) as complementary molecular marker in addition to the 16S rRNA gene for phylogenetic analysis and identification of the species of the family Mycoplasmataceae. Mol. Phylogenet. Evol. 62: 515–528.